Observing the aggregation of proteins in vivo: a new synthetic system shows the "secret" mechanisms of cells
Cells are the basis of the life of any living organism. Their correct functioning is based on a precise internal organisation of space, through which proteins and nucleic acids can perform their task effectively.
Until a few years ago it was believed that cells' internal organisation was due only to the presence of some organelles separated from the rest of the cytoplasm by membranes. Recently, however, it has been discovered that there are a number of organelles, called biomolecular condensates, which have no membrane and play an important role in the cellular homeostasis, since they are able to adapt their structure and function to changes in the internal and external environment of the cell itself. Moreover, the mechanisms underlying the formation of these organelles, mainly composed of proteins and nucleic acids, seem to be also involved in the pathogenesis of diseases caused by abnormal aggregation of proteins such as Alzheimer's, ALS and frontotemporal dementia.
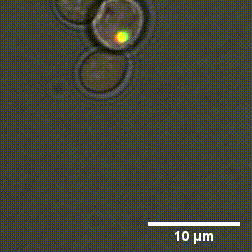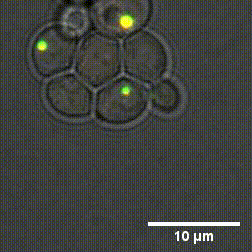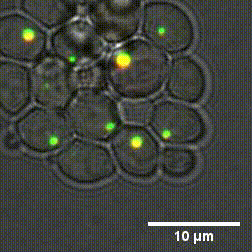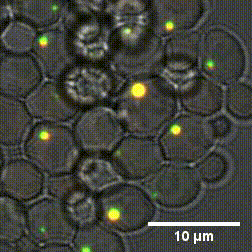
Due to the high number of cytoplasm components, identifying the mechanisms that cause the formation and dissolution of biomolecular condensates under physiological conditions is very difficult. To overcome this problem, a group of researchers from Sapienza, the Weizmann Institute in Tel Aviv and the Universities of Oxford and Vienna have genetically engineered yeast cells to produce proteins capable of forming biomolecular condensates with controllable chemical-physical properties.
"By using cutting-edge experimental methods - says Lorenzo Rovigatti of the Department of Physics of Sapienza University - we were able to understand how the microscopic properties of proteins together with their concentration, control the formation and characteristics of biomolecular condensates." The outcomes of the study, published in the Nature Chemical Biology journal, represent a fundamental step forward in understanding which are the chemical-physical characteristics of proteins that most influence both the physiological and the pathological role of biomolecular condensates.
"The system we have developed is very general - notes Rovigatti - and can also be used to answer questions of great biological relevance about specific microscopic processes, such as the role of the interactions between nucleic acids and proteins, that occur in cells and that are not otherwise observable."
This research represents an essential step towards a better understanding of the protein aggregation mechanisms that is necessary for the cells to function but that, if dysfunctional, can cause the onset of serious diseases.
References:
Designer protein assemblies with tunable phase diagrams in living cells - Meta Heidenreich, Joseph M. Georgeson, Emanuele Locatelli, Lorenzo Rovigatti, Saroj Kumar Nandi, Avital Steinberg, Yotam Nadav, Eyal Shimoni, Samuel A. Safran, Jonathan P. K. Doye, Emmanuel D. Levy - Nature Chemical Biology (2020) https://doi.org/10.1038/s41589-020-0576-z
Further Information
Lorenzo Rovigatti
Department of Physics
lorenzo.rovigatti@uniroma1.it