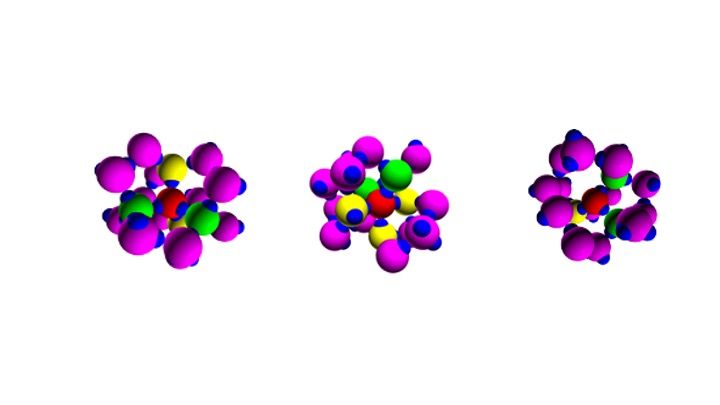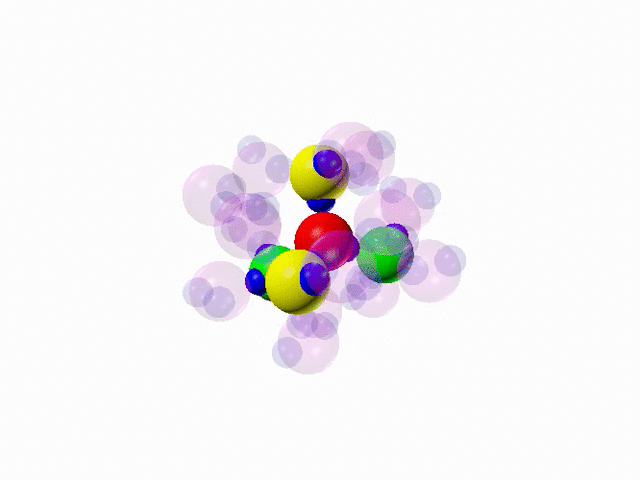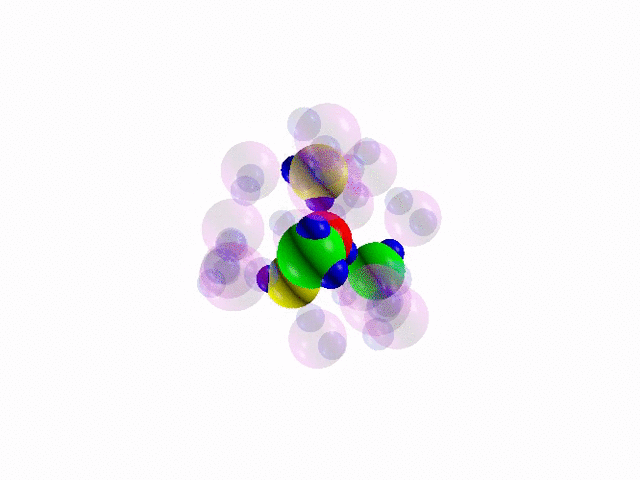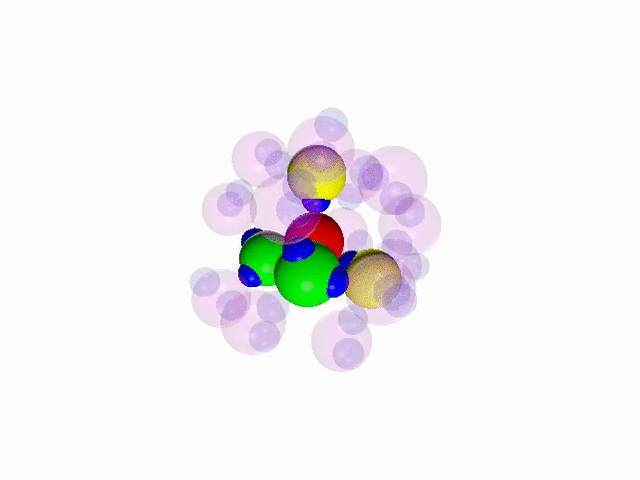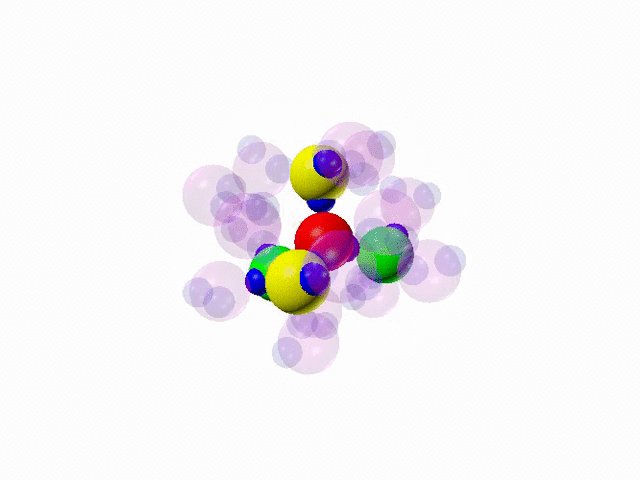The two shapes of water
Each liquid takes the shape of its container. It is a phenomenon we can easily prove by observing it directly with our own eyes. Yet this statement is only valid on a macroscopic level. In fact, at a molecular level, each liquid has its own shape determined by the spatial position in which the molecules composing it are arranged.
Water, the liquid of life, could actually be different and have, in fact, not one, but two different molecular forms: a form in which each molecule is locally surrounded by four other molecules arranged with a tetrahedral (ordered) geometry, forming strong bonds (the hydrogen bonds) with four neighbours, and one in which the tetrahedral structure is significantly distorted, that is a more disordered configuration, in which the central molecule is engaged only in three or five hydrogen bonds.
The competition between these two structures would explain the anomalies of the most precious and abundant element on Earth: indeed water behaves differently from all other liquids existing in nature. For example, as a solid it has a lower density than as a liquid (which explains the buoyancy of ice), it has a very high specific heat (it takes longer than any other liquid to heat up), it has a high surface tension (water drops remain intact on many surfaces, such as on plant leaves, and do not expand like other liquids).
Despite the many theoretical and experimental studies carried out over the last twenty years, no definitive evidence has been produced regarding the role played by these two structures in water.
A new study published on Science provides unequivocal evidence, based on the most accurate models available today, that the uniqueness of water depends precisely on the non-univocity of its local structures. The work is a scientific collaboration between Francesco Sciortino of the Department of Physics at Sapienza University of Rome and Pablo Debenedetti's team at Princeton University (USA). For the first time, research has shown that at very low temperatures the "competition" between the two structures generates two distinct liquid phases with different densities and that the transition between the two "types of water" is a real phase transition, just as it happens, for example, from a solid to a gaseous phase.
In particular, researchers have seen that below a temperature of about 180 Kelvin, the equivalent of -90 degrees Celsius, where water is metastable to ice, the density of the liquid begins to oscillate between two values: low-density liquid and high-density liquid.
"Like ice floating on water," says Francesco Sciortino, "below 180 degrees Kelvin, low-density water floats above high-density water. We have demonstrated, with quite accurate models, a critical point for the liquid-liquid transition: the theoretical proof that was needed to convince the scientific community that it is possible to have a pure system (a single component) with more than one liquid phase".
Extremely long simulations of particularly large systems were needed to achieve these results, a real numerical tour-de-force that required an enormous amount of computing resources, both in Rome and at Princeton. To observe the transition between the two liquids that takes place on a scale of tens of microseconds, before water crystallizes, the authors solved the equations of motion that describe the evolution of the liquid 100 billion times in a row, covering a time interval of about 100 microseconds.
"Thanks to this study - concludes Sciortino - we have a model and accurate numerical data that will allow us to observe the molecular structure on a subnanometric scale in the future, to demonstrate this phase transition experimentally and to discard thermodynamic scenarios that have proved inadequate to grasp its existence".
References:
Second critical point in two realistic models of water - Pablo G. Debenedetti, Francesco Sciortino, Gül H. Zerze - Science 17 Jul 2020 DOI: 10.1126/science.abb9796
Further Information
Francesco Sciortino
Department of Physics
francesco.sciortino@uniroma1.it